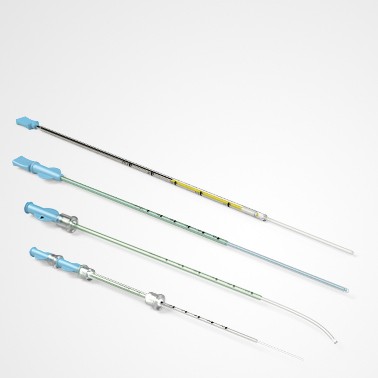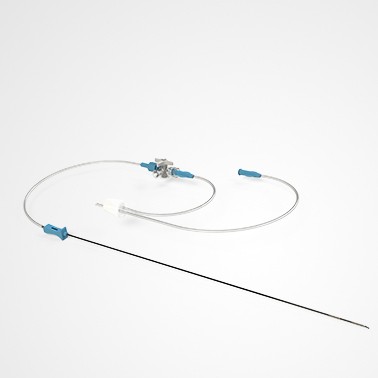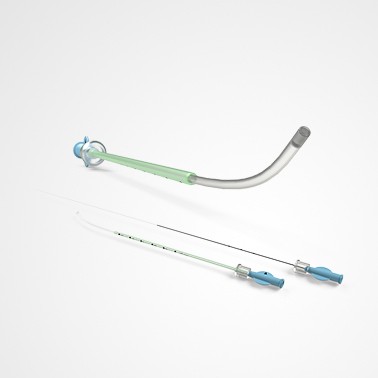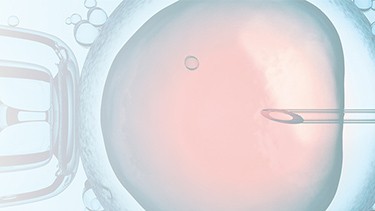

PRINCE MEDICAL, certified NF EN ISO 13485, designs and manufactures a wide range of medical devices (Class IIa and Class I sterile) for Medically Assisted Reproduction : embryo transfer catheters, intrauterine insemination catheter, puncture needle of oocytes, etc. Our PM-LIFE® medical devices are used for intrauterine insemination, vitro fertilisation and oocyte puncture. Perfect control of all industrial processes (moulding, extrusion, assembly of plastic material and steel components, laser cutting, ultrasonic and laser welding, marking, packaging, sterilisation, etc.) is a guarantee of the high level of quality of PRINCE MEDICAL products. The company manufactures and packages its products in a controlled environment and has numerous grey and ISO7 and ISO8 classified clean rooms, guaranteeing a high level of product safety. The regulatory expertise of PRINCE MEDICAL makes it possible to respond perfectly to current standards and to anticipate their evolution.
Medical devices PM
Other Healthcare solutions from the OMERIN Group
Downloads
Medically Assisted Reproduction
INFORMATION

Réservé aux professionnels de santé
Le site www.prince-medical.com permet aux professionnels de santé spécialisés dans les domaines de la Gastro-entérologie, Gynécologie, Procréation Médicalement Assistée de bénéficier d'informations utiles à leur pratique quotidienne.
Reserved for health professionals
The website www.prince-medical.com allows health professionals specialized in the sectors of Gastroenterology, Gynecology, Medically Assisted Reproduction to benefit from information useful to their daily practice.